BioSocial Health J. 1(3):127-135.
doi: 10.34172/bshj.16
Systematic Review
The association of serum phospholipids levels with chronic kidney disease: A systematic review of observational studies
Zahra Shahveghar Asl Conceptualization, Data curation, Formal analysis, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing, 1, 2 
Faezeh Ghalichi Writing – review & editing, 3 
Mohadeseh Badpeyma Writing – review & editing, 1, 2 
Zohreh Ghoreishi Project administration, Supervision, Writing – review & editing, 2, * 
Author information:
1Student Research Committee, Tabriz university of Medical Sciences, Tabriz, Iran
2Department of Clinical Nutrition, Faculty of Nutrition and Food Sciences, Tabriz University of Medical Sciences, Tabriz, Iran
3Department of Nutrition and Food Sciences, Maragheh University of Medical Sciences, Maragheh, Iran
Abstract
Introduction:
Chronic kidney disease (CKD) affects the levels of various metabolites, which may be associated with pathogenesis of chronic diseases. This study aimed to indicate the association between CKD and changes in the levels of phospholipids.
Methods:
This systematic review considered the PRISMA guidelines for reporting the results. We searched the databases MEDLINE (via PubMed), Scopus, Web of Sciences and Google Scholar until June 2023. Case-control and cross-sectional studies investigated the relationship between CKD and alterations of serum levels of phospholipids. We determined the quality of the articles using the modified Newcastle-Ottawa scale (NOS) for cross-sectional studies and the NOS scale for case-control studies.
Results:
A total of 28977 articles were included. One hundred and fifty duplicated articles were removed, 28827 studies were excluded, 343 full-text articles were reviewed and sixteen studies were included at the end. The studies demonstrated a significant association between serum levels of total phospholipids (TPLs), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), lysophosphatidylcholine (LPC), lysophosphatidylethanolamine (LPE), phosphatidic acid (PA) and plasmalogen with renal diseases.
Conclusion:
Phospholipids levels are associated with the kidney diseases. It is important to identify non-invasive ways to diagnose biological risk factors in patients with renal damages, so they can be targeted for early treatment. The included studies reported significant alteration of phospholipids levels in CKD.
Keywords: Phospholipids, Kidney diseases, Systematic reviews
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Introduction
Chronic diseases are global challenges that accounts for eighty percent of all deaths in low- and middle-income countries.1 Chronic kidney disease (CKD) is also a condition in which the kidneys lose their function over time.2 CKD is defined as the existence of renal damages or glomerular filtration rate (GFR) less than 60 mL/min/1.73 m2 for three months or longer without any causes.3 The common risk factors for CKD are diabetes, high blood pressure, inherited kidney disease, heart disease, obesity, and older age.4,5 Due to the prevalence of CKD complications, the search for predictive and diagnostic biomarkers for CKD has attracted considerable interest.6 Several studies have introduced some biomarkers for CKD. Chen et al revealed that glycerophospholipid levels were increased in patients with CKD and the relation between glycerophospholipids and GFR was proved as well.7
Phospholipids comprise one molecule of alcohol, two molecules of fatty acids, and one molecule of phosphate.8 According to the alcohol group in their chemical structures, phospholipids divide into phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), lysophosphatidylethanolamine (LPE), phosphatidylglycerol (PG), phosphatidylinositol (PI), and phosphatidylserine (PS).9 Different physiological and pathological changes of cells have been considered as the underlying mechanisms of the chronic diseases such as diabetes, liver, and kidney diseases that they may result in alterations in the composition and levels of phospholipids in turn.10,11 The distribution patterns of glycerol-PC and several LPC in patients undergoing hemodialysis (HD) were not in the same as healthy people.12 Moreover, plasma phospholipids concentrations were different in patients with early CKD compared to those with patients who were under HD for a long time.13 The comparison of PE, sphingomyelins (SM), and LPC compounds in tumor kidney tissues and normal tissues showed that their serum levels were decreased in renal cancer cells (RCCs).14
However, there are inconsistencies regarding the clinical or trial use of these phospholipids as CKD diagnostic biomarkers. To our knowledge, no systematic review has been done on the association between serum phospholipid levels and kidney diseases. Therefore, the current study was performed to summarize the current evidences regarding the association between serum phospholipids levels and kidney diseases comprehensively.
Material and Methods
Search strategy
The search was conducted using the following MeSH terms and keywords (The search strategy is shown in Table S1):
(((((((((((((((((((((Kidney Diseases[MeSH Terms]) OR (Kidney Diseases[Title/Abstract])) OR (acute kidney injury[Title/Abstract])) OR (Uremia[Title/Abstract])) OR (Azotemia[Title/Abstract])) OR (nephrotic syndrome[Title/Abstract])) OR (diabetic nephropathy[Title/Abstract])) OR (chronic kidney disease[Title/Abstract])) OR (kidney failure[Title/Abstract])) OR (Urea[MeSH Terms])) OR (Blood Urea Nitrogen[MeSH Terms])) OR (Creatinine[MeSH Terms])) OR (Creatinine[Title/Abstract])) OR (Blood Urea Nitrogen[Title/Abstract])) OR (Urea[Title/Abstract])) OR (glomerular filtration rate[Title/Abstract])) OR (glomerular filtration rate[MeSH Terms])) OR (diabetic kidney disease[Title/Abstract])) OR (Proteinuria[MeSH Terms])) OR (Proteinuria[Title/Abstract])) OR (Albuminuria[Title/Abstract])) OR (end stage kidney disease[Title/Abstract]) and (((((((((((((((Phospholipids[MeSH Terms]) OR (Phospholipid[Title/Abstract])) OR (Glycerophospholipid[Title/Abstract])) OR (Glycerylphosphorylcholine[Title/Abstract])) OR (Phosphatidylcholine[Title/Abstract])) OR (phosphatidylcholines[MeSH Terms])) OR (1 2 dipalmitoylphosphatidylcholine[Title/Abstract])) OR (Dimyristoylphosphatidylcholine[Title/Abstract])) OR (Lecithins[Title/Abstract])) OR (glycerol[Title/Abstract])) OR (Phosphatidylethanolamines[Title/Abstract])) OR (Phosphatidylglycerols[Title/Abstract])) OR (Phosphatidylinositols[Title/Abstract])) OR (Phosphatidylserines[Title/Abstract])) OR (serum phospholipid[Title/Abstract])) OR (Lysophosphatidylcholine[Title/Abstract]).
Literature was downloaded to Endnote software to facilitate the review process and manage citations (Version X9; Thomson Reuters, Philadelphia, PA, USA).
In this systematic review, the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines15 (Table S2) were followed. We conducted searches of MEDLINE (PubMed), Scopus, Web of Sciences, and Google Scholar to identify all observational studies evaluating the changes in serum levels of phospholipids and other related metabolites with renal diseases through June 2023, without date restriction.
Eligibility criteria
All original full-text English-language articles that addressed the association between kidney diseases and changes in serum levels of various phospholipids, including total phospholipid (TPL), PC, PE, PS, PI, phosphatidic acid (PA), LPC, lyso PE (LPE) and plasmalogens, were included in this review. Animal, intervention, cohort, and review studies were not included. The review conference publications, book chapters, letters, editorials, posters, commentaries, theses, and studies without available full texts were excluded as well.
Selection of the studies
Three independent reviewers performed a systematic screening of the articles. After removing duplicated articles, the titles and abstracts were checked out based on the inclusion and exclusion criteria by the reviewers, then the full texts of eligible papers were reordered and evaluated, and studies that were unable to meet the predetermined criteria or contained insufficient data were excluded.
Data extraction
Two reviewers extracted the following characteristic cross-sectional independently using a pre-designed data extraction sheet: the first author’s name, year of publication, country, study population, sample size, gender, age, measurement of phospholipids, findings with respect to the relationship between kidney diseases and changes in serum levels of various phospholipids, and some additional information. The third reviewer rechecked the extracted data.
Quality assessment
The quality of the included case control studies was evaluated using the Newcastle-Ottawa scale (NOS).16 NOS scores range from 1 to 9, with higher scores indicating higher-quality research. The risk of bias in a study is considerable if it receives five or fewer stars. The NOS scale has three main sections: selection, comparability, and outcome. Selection comprises four domains: adequate definition of the case, representativeness of the cases, selection of controls, and definition of controls. Comparability was assessed between cases and controls based on study design or data analysis. Exposure consists of three components: Ascertainment of exposure, the same ascertainment methods of cases and controls, and the same non-response rate of cases and controls. Modified NOS was used to assess the quality of the included cross-sectional articles. The modified NOS scale contains three main sections: selection, comparability, and outcome. Selection is composed of four domains: sample representativeness, sample size, exposure ascertainment, and non-respondents. The items in the comparability section are the comparability of subjects in various outcome groups based on the study design or analysis and controlling the confounding factors. The outcome consisted of two sections: evaluation of the outcomes and sufficient follow-up for outcomes to occur.
Results
Study selection
Electronic databases searching PubMed (n = 11 284), Scopus (n = 16 405), Web of Sciences (n = 1288) and Google Scholar yielded a total of 28 977 records in the initial search. After eliminating 150 duplicates, 28 827 studies remained for further screening. During the initial stage, we excluded 28 484 studies based on titles and abstracts, as they were either reviews, cohorts, or animal studies. Then, the full text of the remaining 343 articles was critically assessed, of which 327 articles removed. Finally, the current review included 16 studies that met the eligibility criteria. The flow diagram outlining the selection of the studies is presented in Figure 1. All of the articles recorded at least the change of one metabolite during kidney diseases.
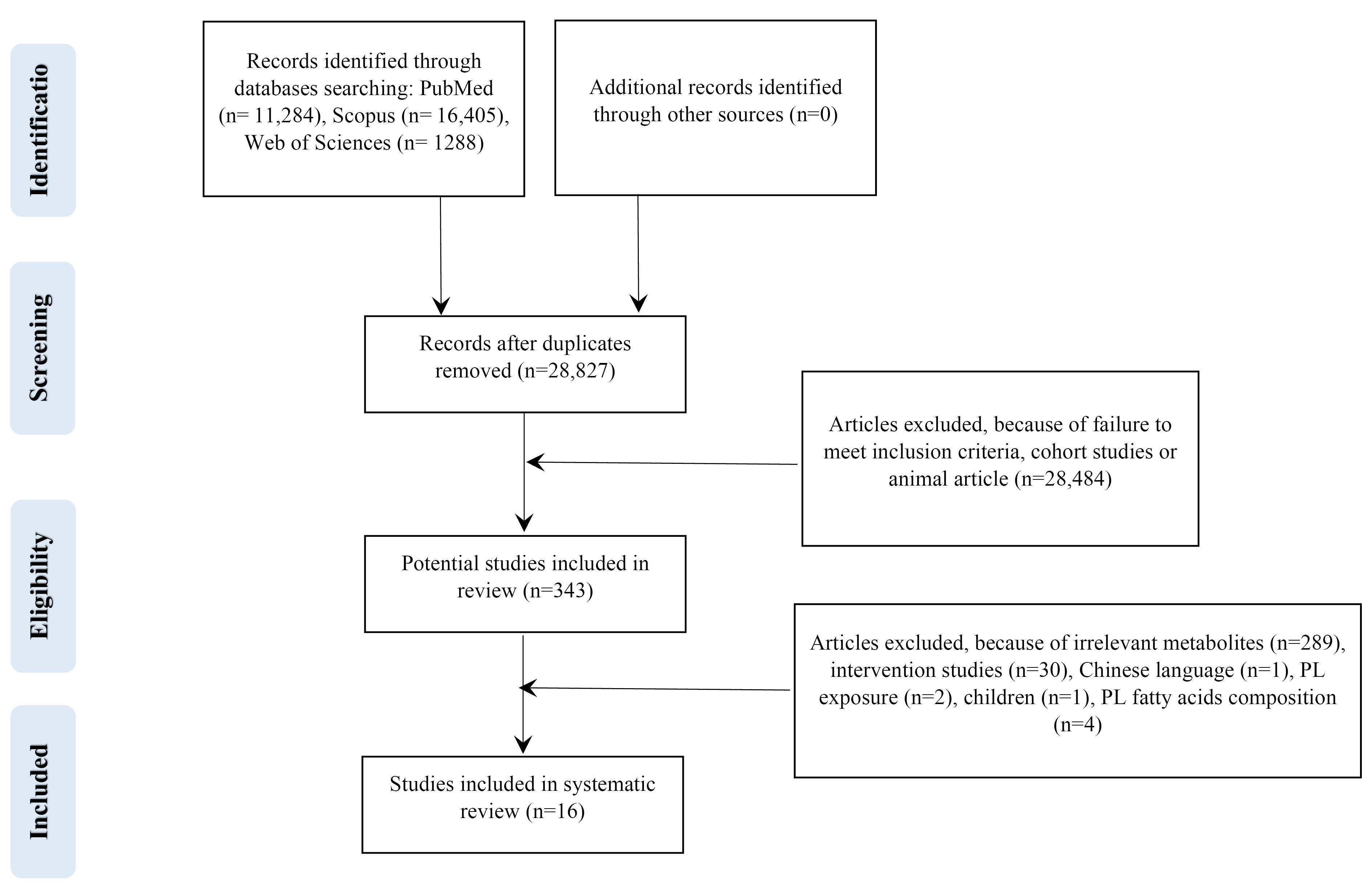
Figure 1.
Flow diagram outlining included studies selection
.
Flow diagram outlining included studies selection
Study characteristics
The articles reviewed in this article were published between 1996 and 2023. Most study populations had renal complications, and both sexes participated in all but two studies, which did not report the exact number of male and female participants.17,18 All studies did not report the follow-up durations, and the sample sizes ranged from 16 to 300. The participants’ mean age ranged from 19.0 to 77.2 years. Only one study did not report the mean age of populations.17 The mean BMI of study subjects was not recorded in most studies, therefore was not included in the data tables. There were 9 studies performed in China,7,12,18-24 two study in Italy25,26 and one study in each of the following countries: Germany,27 USA,28 Emirates,29 France13 and Spain.17
There were 3 studies investigated the relation of metabolome with end-stage renal disease (ESRD),12,24,26 5 study with HD and MHD (maintenance hemodialysis),12,13,25,26,29 3 study with CKD,7,13,22 one study with type 2 diabetes mellitus (T2DM) and diabetic nephropathy (DN),19 2 study with chronic renal failure (CRF),18,27 and one study for acute kidney injury (AKI),21 uremia,17 renal transplant recipients,28 Henoch-Schonlein purpura nephritis (HSPN),20 and renal carcinoma.23
Phospholipids and other related metabolites were measured with thin-layer chromatography (TLC) in one included study,17 Liquid chromatography mass spectrometry (LC–MS) was used in some studies18,28,29 and high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS) assay was performed for analysis of lipidom in two studies.21,22 Other researchers performed gas chromatography (GC)27 two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry (2D LC-QToF MS),24 flow-injection analysis coupled to tandem mass spectrometry (FIA-MS/MS),13 ultra-performance liquid chromatography-high-definition mass spectrometry (UPLC-HDMS),7 normal phase liquid chromatography coupled with time of flight mass spectrometry (NPLC–TOF/MS),19 nuclear magnetic resonance (NMR),23 liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS)12,20 and flow cytometry.25,26 Characteristic of included case-control and cross-sectional studies are reported in Tables 1 and 2, respectively.
Table 1.
Characteristics of case control studies and reported change in levels of methabolites
|
Authers
|
Country
|
Study population
|
Male/
Female
|
Age (Case/
control)
|
Measurement of phospholipids
|
Outcome
|
| Chen et al,12 2020 |
China |
19 patients with ESRD receiving MHD and 12 healthy controls |
18/13 |
56.0/
42.0 |
LC-QTOF-MS |
¯ LPC (16:0), ¯LPC (18:0),
¯LPC (18:3), ¯LPC (20:4),
¯LPC (20:3), ¯LPC (20:5),
¯LPC (16:1) |
| Vecino et al,17 1996 |
Spain |
7 patients with uremia and 9 healthy volunteers |
NR |
41.0 ± 11.0/NR |
TLC |
PC, «LPC, «PI, «PE, «TPL |
| Jia et al,18 2007 |
China |
18 patients with chronic glomerulonephrit and 17 patients with CRF and 18 healthy controls |
NR |
chronic glomerulonephrit: 19-54,
CRF: 24-63/23-61 |
LC-MS |
PE C14:0/C14:0, PC14:0/C14:0,
PS C14: 0/C14:0, LPC C12:0 |
| Zhu et al,19 2011 |
China |
30 T2DM subjects and 52 DN subjects and 30 control subjects |
60/52 |
T2DM: 59.5 ± 7.2, DN: 56.9 ± 8.5/
48.8 ± 5.6 |
NPLC–TOF/MS |
PE (C18:0/C20:4),
¯PI (C18:0/C20:4),
¯PS (C18:0/C18:1),
¯PC (C18:0/C18:0) LPC (C16:0) |
| Zhang et al,20 2021 |
China |
46 HSPN ( + ) patients and 44 HSPN (–) patients |
47/43 |
42.15 ± 18.91/
32.36 ± 14.20 |
LC-Q/TOF-MS |
PE-NMe (16:1/18:3),
PS (14:0/24:0),PC (14:1/P-16:0),
PC (22:6/24:1),PE (P-18:1/14:0),
PS (22:6/24:1),PE (P-16:0e/0:0),
LPC (15:0) |
| Yuan et al,21 2022 |
China |
10 AKI patients and 10 NAKI patients and 10 control subjects |
22/8 |
NAKI:49.2 ± 5.6, AKI:57.9 ± 2.9/
52.6 ± 5.8 |
HPLC-ESI-MS/MS |
PC, PE* |
| Yang et al,22 2013 |
China |
26 consecutive CKD patients and 13 healthy volunteers |
26/13 |
Glomerulonephrit: 41.42 ± 16.44,
Tubulointerstitial injury: 46.71 ± 12.08/ 33.85 ± 14.04 |
HPLC-ESI-MS/MS |
PC 14:1/14:1, PC 16:0/16:0,
PC 16:0/22:3,PC 16:0/18:1,
PC 16:0/18:2 |
| Gao et al,23 2008 |
China |
49 patients with low grade RCC and 25 with advanced RCC and 28 before nephrectomy and 28 patients after nephrectomy and 55 healthy volunteers |
122/63 |
Low grade RCC: 52.0 ± 12.5, Advanced RCC: 62.9 ± 12.9,
before nephrectomy:
54.2 ± 11.3,
after nephrectomy:
54.7 ± 11.1/51.5 ± 13.1 |
NMR |
¯PC/choline |
| Bonomini et al,25 2001 |
Italy |
15 patients with ESRD receiving HD and 15 healthy individuals |
18/12 |
69.0 ± 3.0/
59.0 ± 5.0 |
Flow cytometry |
PS |
| Bonomini et al,26 2004 |
Italy |
16 patients with HD and 16 healthy control subjects |
17/15 |
60.1 ± 2.7/
56.8 ± 2.3 |
Flow cytometry |
PS |
| Brosche et al,27 2002 |
Germany |
30 patients with CRF and 99 normal control subjects |
55/74 |
58.5 ± 12.2/
25.8 - 91.8 |
GC |
¯Plasmalogens |
| Zhao et al,28 2014 |
USA |
27 primary renal transplant recipients |
21/6 |
40.6 ± 9.8/
35.5 ± 8.8 |
LC-MS |
PC, LPC, LPE |
*Changes of these phospholipids were not exactly reported.
Not reported (NR), type 2 diabetes mellitus (T2DM), Diabetic nephropathy (DN), End-Stage Renal Disease (ESRD), phosphatidylserine (PS), maintenance hemodialysis (MHD), chronic renal failure (CRF), liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS), nuclear magnetic resonance (NMR), Total phospholipids (TPL), Chronic Kidney Disease (CKD), high-performance liquid chromatography coupled with electrospray ionization tandem mass spectrometry (HPLC-ESI-MS/MS), Acute kidney injury (AKI), without AKI (NAKI), Henoch-Schonlein purpura nephritis (HSPN), liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-Q/TOF-MS), Liquid chromatography–mass spectrometry (LC-MS), Normal phase liquid chromatography coupled with time of flight mass spectrometry (NPLC–TOF/MS), gas chromatography (GC), phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), lysophosphatidylethanolamine (LPE), phosphatidylinositol (PI), phosphatidylserine (PS), Renal cell carcinoma (RCC), thin-layer chromatography (TLC).
Table 2.
Characteristics of cross-sectional studies and reported change in levels of metabolites
|
Authers
|
Country
|
Study population
|
Male/
Female
|
Age
|
Measurement of phospholipids
|
Outcome
|
| Chen et al,7 2017 |
China |
180 patients with advanced CKD and 120 healthy controls |
173/127 |
Patients;
Discover phase:
57.3 ± 14.5 and Validation phase:
57.1 ± 15.8;
healthy controls;
Discover phase:
55.7 ± 11.2 and Validation phase:
54.1 ± 9.6 |
UPLC-HDMS |
LPC (24:1), LPC (20:4), LPC (18:2), LPC (14:0),
PC (20:2/24:1), PC (22:0/24:0), PC (22:0/14:0), PC (o-18:1/18:2), PC (18:2/22:0), PC (24:0/24:0),
PE (P-18:1/14:1), PE (16:1/18:1), PE (24:1/22:2),PE (22:4/P-18:0), PE (18:4/14:0), PE (24:1/24:1),
PE (24:1/18:1),LPE (18:0), LPE (22:4), LPE (24:1),
LPE (22:1), LPE (16:1), PA (16:0/18:2), |
| Duranton et al,13 2018 |
France |
77 patients with CKD and HD |
49/28 |
CKD2-3:
64.4 (54.4, 75.2), CKD4-5:
75.9 (69.1, 79.3)
HD: 77.2 (68.2, 82.1) |
FIA-MS/MS |
¯PC (34:4), ¯PC (34:2), ¯PC (34:3), ¯PC (36:3), ¯PC (36:4), ¯PC (36:5), ¯PC (36:6), ¯PC (38:1), ¯PC (38:2), ¯PC (38:3), ¯PC (40:1), ¯PC (40:4), ¯PC (42:2), ¯PC (42:3), ¯PC (42:4), ¯PC (44:4), ¯PC (44:5), ¯LPC (14:0), ¯LPC (16:0), ¯LPC (16:1), ¯LPC (18:0), ¯LPC (18:1), ¯LPC (18:2), ¯LPC (20:3),
¯LPC (20:4) |
| Tang et al,24 2014 |
China |
20 stable ESRD patients |
10/10 |
PD technical
Survival: 56.93 ± 14.50,
PD technical
Failure: 65.00 ± 11.73 |
2D LC-QToF MS |
«PS (41:4), «PI (40:4), «PC (35:1), «PC (2:11),
«PC (42:9), «PE (14:0/14:0), «PC (14:1/14:1), «LPC (17:0), «LPE (17:1) |
| Banimfreg et al,29 2022 |
Emirati |
11 HD subjects with and 25 patients without diabetes |
16/20 |
females:
69.9 ± 8.16
and males:
73.68 ± 13.07 |
LC-MS |
*PC (16:0/16:0), *PC (18:1/18:1), |
*Changes of these phospholipids were not exactly reported. hemodialysis (HD), Liquid Chromatography-Mass Spectrometry (LC-MS), ultra-performance liquid chromatography-high-definition mass spectrometry (UPLC-HDMS), Chronic Kidney Disease (CKD), flow-injection analysis coupled to tandem mass spectrometry (FIA-MS/MS), end-stage renal disease (ESRD), peritoneal dialysis (PD), two-dimensional liquid chromatography-quadrupole time-of-flight mass spectrometry (2D LC-QToF MS), phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE), lysophosphatidylethanolamine (LPE), phosphatidylinositol (PI), phosphatidylserine (PS), Phosphatidic acid (PA).
Quality assessment
Methodological quality assessment for case-control and cross-sectional studies were shown in Tables 3 and 4 respectively. Decision about the risk of bias for each item in all included case-control and cross-sectional studies were reported as score of stars. A minor risk of bias was shown based on the results of the studies.
Table 3.
Risk of bias indicating case control studies’ quality assessment at an individual level
|
Study
|
Selection
|
Comparability
|
Exposure
|
Total
|
|
Adequate definition of the case
|
Representativeness of the cases
|
Selection of controls
|
Definition of controls
|
Comparability of cases and controls
|
Ascertainment of exposure
|
Cases and controls: same ascertainment method
|
Cases and controls: same non-response rate
|
|
Design
|
Analysis
|
| Chen et a,12 2020 |
* |
* |
* |
* |
- |
- |
* |
- |
- |
5/9 |
| Vecino et al,17 1996 |
* |
* |
- |
* |
* |
* |
* |
- |
- |
6/9 |
| Jia et al,18 2007 |
* |
* |
- |
* |
* |
- |
* |
* |
- |
6/9 |
| Zhu et al,19 2011 |
* |
* |
- |
* |
- |
- |
* |
* |
- |
5/9 |
| Zhang et al,20 2021 |
* |
* |
* |
* |
* |
- |
* |
* |
- |
7/9 |
| Yuan et al,21 2022 |
* |
* |
* |
* |
* |
* |
* |
* |
- |
8/9 |
| Yang et al,22 2013 |
* |
* |
- |
* |
* |
- |
* |
* |
- |
6/9 |
| Gao et al,23 2008 |
* |
* |
* |
* |
- |
- |
* |
* |
- |
6/9 |
| Bonomini et al,25 2001 |
* |
* |
- |
* |
* |
* |
* |
- |
- |
6/9 |
| Bonomini et al,26 2004 |
* |
* |
- |
* |
* |
* |
* |
* |
- |
7/9 |
| Brosche et al,27 2002 |
* |
* |
- |
- |
* |
* |
- |
* |
- |
5/9 |
| Zhao et al,28 2014 |
* |
* |
* |
* |
* |
* |
* |
* |
- |
8/9 |
Table 4.
Risk of bias indicating cross sectional studies’ quality assessment at an individual level
|
Study
|
Selection
|
Comparability
|
Outcome
|
Total
|
|
Representativeness of the sample
|
Sample size
|
Ascertainment of exposure
|
Non-respondents
|
Design
|
Analysis
|
Assessment of outcome
|
Adequacy of follow up
|
| Chen et al,7 2017 |
* |
- |
* |
- |
* |
* |
* |
* |
6/8 |
| Duranton et al,13 2019 |
* |
- |
* |
- |
* |
* |
* |
- |
5/8 |
| Tang et al,24 2014 |
* |
- |
* |
* |
* |
* |
* |
* |
7/8 |
| Banimfreg et al,29 2022 |
* |
* |
- |
- |
* |
* |
* |
- |
5/8 |
Metabolite changes in the renal diseases
Changes in different metabolites, such as phospholipids and other related lipid species in various renal diseases, in case-control studies were reported in Table 1. Table 2 presents lipidome profiling in kidney diseases for cross-sectional studies.
Total phospholipid
Only one case-control study reported the change in TPLs among the patients with uremia and healthy volunteers; they have seen an increased amount of TPLs in uremic conditions.17
Phosphatidylcholine
Phosphatidylcholines were measured in seven case-control studies on renal malfunction; one study in each of the following conditions: ESRD patients receiving MHD,12 people with kidney carcinoma,23 CRF patients,18 population with uremia,17 HSPN patients,20 renal transplant recipients,28 and DN.19 Two cross-sectional studies were on patients with CKD,7,13 ESRD patients24 and those who receiving HD.29 Gao et al reported a decrease in PC/choline ratio in patients with advanced RCCs compared with healthy controls.23 Rise in PCs levels were found in different renal disorders such as CRF,18 uremia,17 CKD,22 and HSPN.20 In study subjects with T2DM and DN, a reduction of PCs levels was observed.19 One case-control study among patients with kidney diseases did not report explicit changes of PCs levels.21 Table 2 showed that the levels of PCs were high in CKD patients.30 Duranton et al reported that the PCs levels were decreased in CKD patients with non-alcoholic fatty liver disease, receiving HD.13,31 No significant changes were reported in the study by Tang et al in ESRD population24 and one cross-sectional study did not report the exact results of PCs levels.29
Phosphatidylethanolamine
Measurements of serum levels of PEs were reported in five case-control studies of renal disease. It was seen that the levels of PEs were high in three studies in patients with CRF, HSPN, and DN compared to control groups.18-20 Vecino et al did not report any changes in the PE levels among uremic patients17 and Yuan et al also did not report any exact change of PE levels,21 however, it was measured in two cross-sectional studies in CKD, and ESRD patients.7,24 Tang et al reported that PE levels were similar in two groups of patients undergoing peritoneal dialysis (PD): technical survival and technical failure,24 while there was a significant increase in the levels of PE in patients with advanced CKD compared with healthy controls.7
Phosphatidylserine
The PS levels were measured in five case-control studies on patients with renal diseases and one cross-sectional study on those with ESRD. Among case-control studied, four of them reported that PS levels increased during kidney damages,18,20,25,26 while one study showed its reduction in patients with T2DM and DN.19 Notably, one cross-sectional study could not see any changes in ESRD patients receiving PD.24
Phosphatidylinositol
Zhu et al observed low serum levels of PI in T2DM and DN subjects compared with the control subjects in a case-control study.19 In another study, serum concentrations of PIs in patients with uremia and healthy volunteers were similar.17 PI serum levels were measured in two cross-sectional studies on ESRD patients, showed reduced PI levels32 whereas, Tang et al reported no significant changes in this regard.24
Phosphatidic acid
Only one included cross-sectional study measured the levels of PAs that it showed the increased levels.7
Lysophosphatidylcholine
Six renal case-control studies measured serum LPC levels in kidney diseases (Table 1), four of them reported the increased levels18-20,28 while Vecino et al could not see any change in LPC levels.17 Of note, Chen et al observed a reduction in serum levels of LPC in a case-control study.12 Three cross-sectional studies investigated the serum change of LPC levels, one showed an increase,22 one demonstrated a decrease,13 while Tang et al observed no change in LPC levels.24
Lysophosphatidylethanolamine
One case-control study identified an increase in LPE levels among renal transplant recipients.28 Chen et al investigated the LPE levels in advanced CKD and healthy controls and results showed an increase.7 Another cross-sectional study on patients with renal disorders reported no difference of LPE levels among participants.24
Plasmalogen
It was reported that the levels of plasmalogens were reduced in patients with CRF compared to normal controls.27
Discussion
In the current study, twelve case-control, and four cross-sectional studies which assessed the association between serum levels of phospholipids with the renal disorders were reviewed systematically. There were significant correlations between serum levels of TPL, PC, PE, PS, PI, PA, LPC, LPE, and plasmalogen with CKD.
It is important to identify non-invasive ways to find biological risk factors of renal diseases, so they can be targeted for early treatment. Also, the results implied a better understanding of how phospholipids dysregulation may relate to the increased rate of the kidney diseases. This study also contributes to the better identification of potential phospholipid biomarkers that may help to improve the detection and prevention of biological renal damages.
Metabolism of phospholipids is abnormal among patients with kidney diseases including uremia, chronic nephritis, CKD and CRF.18 Mechanism for high levels of phospholipids in chronic glomerulonephritis was described as phospholipase C/protein kinase C (PKC) signal transduction pathways. First step was cleavage of PI into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol.33 Inositol trisphosphate led to secretion of the Calcium ion (Ca2+) and then high levels of Ca2+ was seen.34,35 PKC become active that leads to decreasing the space between endothelial cells 36 and changing the structure and function of phosphorylated actin.37 Finally, the activation of PKC causes the damage of the carriers of endothelial cells in renal glomerular capillaries and, therefore, the permeability of the vessels rises. Protein and erythrocytes may infiltrate; thus, proteinuria as well as hematuria occur. Also, PKC can induce high angiotensin levels,38 hypertension and chronic glomerulonephritis thereafter.
Costello et al reported that among uremic patients, plasma levels of phospholipase A2 (PLA-2) were high.39 Phospholipase A2 (PLA-2) could synthesize Lyso-phospholipids from phospholipids. There were evidences indicating the decreased plasma levels of LPC in patients with uremia.17,40,41
It was shown that lecithin-cholesterol acyltransferase (LCAT) abnormality was the main reason of increased PC levels in uremic patients.41,42 In a rat model study, Zhao et al predicted CRF through phospholipids biomarkers such as increased ratio of PC 16:0/18:2 as well as serum levels of LPCs.43
Researchers have demonstrated a direct association between urinary phospholipids and proteinuria, and serum levels of PCs and creatinine. Therefore PCs and SMs could be used as CKD biomarkers.22
High levels of PE among patients with HSPN implied that kidney function was decreased through cell apoptosis induced by PE.20 Also, it was indicated that PE can lead to apoptosis of liver cells via the B-cell lymphoma protein 2 (Bcl-2)-associated X (Bax) pathway.44
Compared to early CKD stages, LPCs in most HD patients decreased, and they tended to have lower levels compared to healthy controls in other investigations.13 Although antibacterial, antioxidative, and antiatherogenic properties have also been noted, LPCs primarily operate on G protein-coupled receptors and exhibit proatherogenic activity.45 Both PLA-2 activity and LCAT deficiency have been documented in CKD patients (LPCs are derived from PCs due to LCAT and PLA-2 activity).46,47
Zhao et al observed that after kidney transplantation, the non-acute graft rejection group had the higher levels of certain lipids (carnitines, choline, various polyunsaturated fatty acids, SMs, PCs, LPCs, and LPEs), as well as the increased concentration of gut microbiota-associated metabolites (indoxyl sulfate, p-cresol sulfate, and hippuric acid).28 The lower serum levels of LPCs, LPEs, and polyunsaturated fatty acids in the acute graft rejection group may potentially be due to high-dose immunosuppressant’s that in turn, decreased PLA-2 activity. Additionally, a substantial decrease in SM species was seen in the serum of the patients who had acute graft transplant rejection. SM deficiency may change lipid raft formation and increase the risk of oxidative damage.48,49
Zhu et al found that two novel biomarkers, i.e., PI C18:0/22:6 and SM dC18:0/20:2, can be utilized to distinguish between healthy individuals, T2DM cases, and DN cases from each other. We generated the phospholipids in the following elution orders: PEs, PGs, PIs, PSs, PCs, SMs, and LPCs. LPCs, PEs, PG, SMs, one PC species, and one PI species were all up-regulated in patients compared to controls, while PE, PS, and two PC species were down-regulated.19
Generally, chronic diseases, such as CKD, have special bio-psycho-social aspects. Early prediction and treatment of psychiatric disorders such as depression and anxiety contribute to the prevention of renal disorders. Early prediction can improve social support such as financial assistance and rehabilitation to perform usual activities (doing the job, traveling, leisure, and sports.
Relatively high number of included studies was the strength of this review, which make it possible to generalize the results. Additionally, the quality of the studies was evaluated according to the NOS, and majority of the studies were determined as having high-quality. However, studies’ heterogeneity, especially in outcomes and methods of analysis, was a known limitation of the current study that prevented preparing a meta-analysis. Moreover, removing non-English studies may cause the language bias.
Conclusion
Association of the serum phospholipids levels with renal dysfunction was observed in this systematic review. Levels of phospholipids in patients with CKD in comparison to the healthy individuals were different, therefore, it can be considered as a diagnostic biomarker for early detection and prevention of advanced renal diseases.
Acknowledgements
The research protocol was approved and supported by Student Research Committee, Tabriz University of Medical Sciences (registration code: 72168).
Competing Interests
The authors state that they have no conflict of interest and no competing interests.
Ethical Approval
Not applicable.
Supplementary Files
Supplementary file 1 Contain Table S1 and S2.
(pdf)
References
- World Health Organization (WHO). World Health Statistics 2012. Geneva, Switzerland: WHO; 2012.
- Parmar MS. Chronic renal disease. BMJ 2002; 325(7355):85-90. doi: 10.1136/bmj.325.7355.85 [Crossref] [ Google Scholar]
- Chapter 1: definition and classification of CKD. Kidney International Supplements 2013;3(1):19-62. 10.1038/kisup.2012.64.
- Luyckx VA, Cherney DZI, Bello AK. Preventing CKD in developed countries. Kidney Int Rep 2020; 5(3):263-77. doi: 10.1016/j.ekir.2019.12.003 [Crossref] [ Google Scholar]
- Ngendahayo F, Mukamana D, Ndateba I, Nkurunziza A, Adejumo O, Chironda G. Chronic kidney disease (CKD): knowledge of risk factors and preventive practices of CKD among students at a University in Rwanda. Rwanda J Med Health Sci 2019; 2(2):185-93. doi: 10.4314/rjmhs.v2i2.15 [Crossref] [ Google Scholar]
- Niewczas MA, Mathew AV, Croall S, Byun J, Major M, Sabisetti VS. Circulating modified metabolites and a risk of ESRD in patients with type 1 diabetes and chronic kidney disease. Diabetes Care 2017; 40(3):383-90. doi: 10.2337/dc16-0173 [Crossref] [ Google Scholar]
- Chen H, Chen L, Liu D, Chen DQ, Vaziri ND, Yu XY. Combined clinical phenotype and lipidomic analysis reveals the impact of chronic kidney disease on lipid metabolism. J Proteome Res 2017; 16(4):1566-78. doi: 10.1021/acs.jproteome.6b00956 [Crossref] [ Google Scholar]
- Kucerka N, Pencer J, Sachs JN, Nagle JF, Katsaras J. Curvature effect on the structure of phospholipid bilayers. Langmuir 2007; 23(3):1292-9. doi: 10.1021/la062455t [Crossref] [ Google Scholar]
- Tatulian SA. Ionization and ion binding. In: Cevc G, ed. Phospholipid Handbook. New York: Marcel Dekker; 1993. p. 511-52.
- Sonoki K, Iwase M, Iino K, Ichikawa K, Ohdo S, Higuchi S. Atherogenic role of lysophosphatidylcholine in low-density lipoprotein modified by phospholipase A2 and in diabetic patients: protection by nitric oxide donor. Metabolism 2003; 52(3):308-14. doi: 10.1053/meta.2003.50049 [Crossref] [ Google Scholar]
- Han X, D MH, McKeel DW Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: potential role in disease pathogenesis. J Neurochem 2002; 82(4):809-18. doi: 10.1046/j.1471-4159.2002.00997.x [Crossref] [ Google Scholar]
- Chen Y, Wen P, Yang J, Niu J. Plasma metabolomics profiling in maintenance hemodialysis patients based on liquid chromatography quadrupole time-of-flight mass spectrometry. Kidney Dis (Basel) 2020; 6(2):125-34. doi: 10.1159/000505156 [Crossref] [ Google Scholar]
- Duranton F, Laget J, Gayrard N, Saulnier-Blache JS, Lundin U, Schanstra JP, et al. The CKD plasma lipidome varies with disease severity and outcome. J Clin Lipidol 2019;13(1):176-85.e8. 10.1016/j.jacl.2018.07.010.
- Cífková E, Holčapek M, Lísa M, Vrána D, Melichar B, Študent V. Lipidomic differentiation between human kidney tumors and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2015; 1000:14-21. doi: 10.1016/j.jchromb.2015.07.011 [Crossref] [ Google Scholar]
- Golder S, Loke YK, Bland M. Meta-analyses of adverse effects data derived from randomised controlled trials as compared to observational studies: methodological overview. PLoS Med 2011; 8(5):e1001026. doi: 10.1371/journal.pmed.1001026 [Crossref] [ Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2000.
- Vecino A, Teruel JL, Navarro JL, Cesar JM. Plasma phospholipids and platelet function in uremic patients. Am J Nephrol 1996; 16(5):409-11. doi: 10.1159/000169033 [Crossref] [ Google Scholar]
- Jia L, Wang C, Zhao S, Lu X, Xu G. Metabolomic identification of potential phospholipid biomarkers for chronic glomerulonephritis by using high performance liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2007; 860(1):134-40. doi: 10.1016/j.jchromb.2007.10.033 [Crossref] [ Google Scholar]
- Zhu C, Liang QL, Hu P, Wang YM, Luo GA. Phospholipidomic identification of potential plasma biomarkers associated with type 2 diabetes mellitus and diabetic nephropathy. Talanta 2011; 85(4):1711-20. doi: 10.1016/j.talanta.2011.05.036 [Crossref] [ Google Scholar]
- Zhang Q, Lai LY, Cai YY, Wang MJ, Ma G, Qi LW. Serum-urine matched metabolomics for predicting progression of Henoch-Schonlein purpura nephritis. Front Med (Lausanne) 2021; 8:657073. doi: 10.3389/fmed.2021.657073 [Crossref] [ Google Scholar]
- Yuan H, Gao Z, Chen G, Peng C, Sun Y, Jiang B. An integrative proteomics metabolomics based strategy reveals the mechanisms involved in wasp sting induced acute kidney injury. Toxicon 2022; 205:1-10. doi: 10.1016/j.toxicon.2021.11.005 [Crossref] [ Google Scholar]
- Yang WL, Bai Q, Li DD, A TL, Wang S, Zhao RS. Changes of urinary phospholipids in the chronic kidney disease patients. Biomarkers 2013; 18(7):601-6. doi: 10.3109/1354750x.2013.837100 [Crossref] [ Google Scholar]
- Gao H, Dong B, Liu X, Xuan H, Huang Y, Lin D. Metabonomic profiling of renal cell carcinoma: high-resolution proton nuclear magnetic resonance spectroscopy of human serum with multivariate data analysis. Anal Chim Acta 2008; 624(2):269-77. doi: 10.1016/j.aca.2008.06.051 [Crossref] [ Google Scholar]
- Tang W, Li M, Lu XH, Liu HW, Wang T. Phospholipids profiling and outcome of peritoneal dialysis patients. Biomarkers 2014; 19(6):505-8. doi: 10.3109/1354750x.2014.943290 [Crossref] [ Google Scholar]
- Bonomini M, Sirolli V, Reale M, Arduini A. Involvement of phosphatidylserine exposure in the recognition and phagocytosis of uremic erythrocytes. Am J Kidney Dis 2001; 37(4):807-14. doi: 10.1016/s0272-6386(01)80130-x [Crossref] [ Google Scholar]
- Bonomini M, Dottori S, Amoroso L, Arduini A, Sirolli V. Increased platelet phosphatidylserine exposure and caspase activation in chronic uremia. J Thromb Haemost 2004; 2(8):1275-81. doi: 10.1111/j.1538-7836.2004.00837.x [Crossref] [ Google Scholar]
- Brosche T, Platt D, Knopf B. Decreased concentrations of serum phospholipid plasmalogens indicate oxidative burden of uraemic patients undergoing haemodialysis. Nephron 2002; 90(1):58-63. doi: 10.1159/000046315 [Crossref] [ Google Scholar]
- Zhao X, Chen J, Ye L, Xu G. Serum metabolomics study of the acute graft rejection in human renal transplantation based on liquid chromatography-mass spectrometry. J Proteome Res 2014; 13(5):2659-67. doi: 10.1021/pr5001048 [Crossref] [ Google Scholar]
- Banimfreg BH, Alshraideh H, Shamayleh A, Guella A, Semreen MH, Al Bataineh MT. Untargeted metabolomic plasma profiling of Emirati dialysis patients with diabetes versus non-diabetic: a pilot study. Biomolecules 2022; 12(7):962. doi: 10.3390/biom12070962 [Crossref] [ Google Scholar]
- Wang ZH, Zheng KI, Wang XD, Qiao J, Li YY, Zhang L. LC-MS-based lipidomic analysis in distinguishing patients with nonalcoholic steatohepatitis from nonalcoholic fatty liver. Hepatobiliary Pancreat Dis Int 2021; 20(5):452-9. doi: 10.1016/j.hbpd.2021.05.008 [Crossref] [ Google Scholar]
- Arendt BM, Ma DW, Simons B, Noureldin SA, Therapondos G, Guindi M. Nonalcoholic fatty liver disease is associated with lower hepatic and erythrocyte ratios of phosphatidylcholine to phosphatidylethanolamine. Appl Physiol Nutr Metab 2013; 38(3):334-40. doi: 10.1139/apnm-2012-0261 [Crossref] [ Google Scholar]
- Krautbauer S, Meier EM, Rein-Fischboeck L, Pohl R, Weiss TS, Sigruener A. Ceramide and polyunsaturated phospholipids are strongly reduced in human hepatocellular carcinoma. Biochim Biophys Acta 2016; 1861(11):1767-74. doi: 10.1016/j.bbalip.2016.08.014 [Crossref] [ Google Scholar]
- Rhee SG. Regulation of phosphoinositide-specific phospholipase C. Annu Rev Biochem 2001; 70:281-312. doi: 10.1146/annurev.biochem.70.1.281 [Crossref] [ Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science 2002; 296(5573):1655-7. doi: 10.1126/science.296.5573.1655 [Crossref] [ Google Scholar]
- Downes CP, Gray A, Lucocq JM. Probing phosphoinositide functions in signaling and membrane trafficking. Trends Cell Biol 2005; 15(5):259-68. doi: 10.1016/j.tcb.2005.03.008 [Crossref] [ Google Scholar]
- Lynch JJ, Ferro TJ, Blumenstock FA, Brockenauer AM, Malik AB. Increased endothelial albumin permeability mediated by protein kinase C activation. J Clin Invest 1990; 85(6):1991-8. doi: 10.1172/jci114663 [Crossref] [ Google Scholar]
- Xia P, Aiello LP, Ishii H, Jiang ZY, Park DJ, Robinson GS. Characterization of vascular endothelial growth factor’s effect on the activation of protein kinase C, its isoforms, and endothelial cell growth. J Clin Invest 1996; 98(9):2018-26. doi: 10.1172/jci119006 [Crossref] [ Google Scholar]
- Kang N, Alexander G, Park JK, Maasch C, Buchwalow I, Luft FC. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int 1999; 56(5):1737-50. doi: 10.1046/j.1523-1755.1999.00725.x [Crossref] [ Google Scholar]
- Costello J, Franson RC, Landwehr K, Landwehr DM. Activity of phospholipase A2 in plasma increases in uremia. Clin Chem 1990; 36(2):198-200. [ Google Scholar]
- Schardt W, Wardle EN. Plasma lysolecithin in uraemia. Clin Chim Acta 1975; 63(2):223-5. doi: 10.1016/0009-8981(75)90166-7 [Crossref] [ Google Scholar]
- Gillett MP, Teixeira V, Dimenstein R. Decreased plasma lecithin:cholesterol acyltransfer and associated changes in plasma and red cell lipids in uraemia. Nephrol Dial Transplant 1993; 8(5):407-11. [ Google Scholar]
- Appel G. Lipid abnormalities in renal disease. Kidney Int 1991; 39(1):169-83. doi: 10.1038/ki.1991.22 [Crossref] [ Google Scholar]
- Zhao YY, Cheng XL, Wei F, Xiao XY, Sun WJ, Zhang Y. Serum metabonomics study of adenine-induced chronic renal failure in rats by ultra performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. Biomarkers 2012; 17(1):48-55. doi: 10.3109/1354750x.2011.637180 [Crossref] [ Google Scholar]
- Yao Y, Huang C, Li ZF, Wang AY, Liu LY, Zhao XG. Exogenous phosphatidylethanolamine induces apoptosis of human hepatoma HepG2 cells via the bcl-2/Bax pathway. World J Gastroenterol 2009; 15(14):1751-8. doi: 10.3748/wjg.15.1751 [Crossref] [ Google Scholar]
- Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis 2010; 208(1):10-8. doi: 10.1016/j.atherosclerosis.2009.05.029 [Crossref] [ Google Scholar]
- Papavasiliou EC, Gouva C, Siamopoulos KC, Tselepis AD. Erythrocyte PAF-acetylhydrolase activity in various stages of chronic kidney disease: effect of long-term therapy with erythropoietin. Kidney Int 2005; 68(1):246-55. doi: 10.1111/j.1523-1755.2005.00399.x [Crossref] [ Google Scholar]
- Gillett MP, Obineche EN, Lakhani MS, Abdulle AM, Amirlak I, Al Rukhaimi M. Levels of cholesteryl esters and other lipids in the plasma of patients with end-stage renal failure. Ann Saudi Med 2001; 21(5-6):283-6. doi: 10.5144/0256-4947.2001.283 [Crossref] [ Google Scholar]
- Peña LA, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol 1997; 53(5):615-21. doi: 10.1016/s0006-2952(96)00834-9 [Crossref] [ Google Scholar]
- Ballou LR, Laulederkind SJ, Rosloniec EF, Raghow R. Ceramide signalling and the immune response. Biochim Biophys Acta 1996; 1301(3):273-87. doi: 10.1016/0005-2760(96)00004-5 [Crossref] [ Google Scholar]