BioSocial Health J. 1(3):136-145.
doi: 10.34172/bshj.36
Original Article
Daily brisk walking increases intestinal acetic acid in short-chain fatty adults in healthy young adults: A randomized controlled trial
Emiko Morita Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing, 1, * 
Suguru Ando Formal analysis, Investigation, Supervision, Writing – review & editing, 1
Yumi Fukuyama Investigation, Writing – review & editing, 1
Yui Kikuchi Investigation, Writing – review & editing, 1
Sho Kumabe Investigation, Writing – review & editing, 1
Yoshihiro Yamashina Investigation, Supervision, Writing – review & editing, 1
Author information:
1Department of Physical Therapy Faculty of Health Science, Aino University, 4-5-4 Higashioda, Ibaraki city, Osaka 567-0012, Japan
Abstract
Introduction:
In this study, we aimed to investigate the effect of daily physical activity levels, specifically brisk walking, on short-chain fatty acid (SCFA) production through the modulation of SCFA-producing bacteria in young individuals.
Methods:
This 12-week randomized comparative trial included 30 participants. The participants were assigned to either the group that walked 8000 steps daily, including 20 minutes of brisk walking at an intensity of≥5 metabolic equivalents (BW group), or the group that walked 8000 steps daily at their customary pace (CP group). The SCFA levels and composition of the intestinal microbiota were assessed before and after the intervention. Daily physical activity and cardiorespiratory fitness were monitored using an accelerometer and the incremental shuttle walking test (ISWT), respectively.
Results:
The BW group exhibited a significant increase in the level of acetic acid, a type of SCFA, following the intervention. The relative abundance of intestinal Bifidobacterium was significantly higher in participants who successfully completed 20 minutes of brisk walking during the intervention.
Conclusion:
Daily brisk walking for 20 minutes enhances acetic acid production by fostering the proliferation of Bifidobacterium in young individuals.
Keywords: Bifidobacterium, Gut microbiota, Physical activity, Short-chain fatty acids
Copyright and License Information
© 2024 The Author(s).
This is an open access article distributed under the terms of the Creative Commons Attribution License (
http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Funding Statement
This work was supported by JSPS KAKENHI (grant number 21K17521).
Introduction
The gut microbiota is associated with various diseases,1 and short-chain fatty acids (SCFAs) produced by gut bacteria are vital in maintaining health.2-5 In Japan, the packaging of yogurt products mentions the phrase “the power to create SCFA,” indicating that the term SCFA has permeated daily lives.
SCFAs, primarily including acetic, butyric, and propionic acid, are metabolites produced by SCFA-producing bacteria in the intestine via fermentation.6 They have diverse physiological actions, and play various useful roles in the body, including regulation of host energy metabolism,2 anti-inflammatory action,3 inhibition of fat accumulation,4 and regulation of neurotransmitters and neurotrophic factors.5 Dietary fiber and oligosaccharides are the raw materials for producing SCFA; moreover, SCFA-producing bacteria, such as Bifidobacterium are found in fermented foods, such as yogurt.7 In addition to nutrition, physical activity and exercise can increase SCFA levels or SCFA-producing bacteria. In non-athletes, increased physical activity affects the SCFA content,7 and in sedentary middle-aged insulin-resistant participants, moderate exercises such as 40–60 minutes of cycling providing 60% of maximal oxygen intake increases SCFA-producing bacteria, such as Faecalibacterium and Lachnospira spp.8 Furthermore, in college-level swimmers, Coprococcus spp. decrease with decreasing training,9 whereas Roseburia hominis10 and Faecalibacterium spp. decrease with increased physical activity and exercise intensity.9 However, few studies have reported the effects of increasing daily physical activity on SCFA and SCFA-producing bacteria in young non-exercising adults. Therefore, we tested the effect of brisk walking for 3 months on the SCFA levels in the guts of young individuals. We hypothesized that brisk walking would increase the SCFA levels by increasing the number of SCFA-producing bacteria.
Methods
Participants
We recruited 30 healthy individuals aged 20–23 years who had no exercise habits, through advertisements posted at Aino University. We determined the sample size for this study based on a previous study on gut microbiota and brisk walking.11 Participants were assigned numerical identifiers according to the sequence of their recruitment. Their health status was assessed using structured interviews. Applicants with a history of musculoskeletal, cardiovascular, or neurological disorders were excluded. Participants who did not agree to walk at least 8000 steps daily were also excluded. Finally, 30 participants were enrolled.
This study was registered in the Information Network Clinical Trials Registry (UMIN000051961). Written informed consent was obtained from all participants. The study protocol conformed with the ethical guidelines of the 1975 Declaration of Helsinki.
Study design
This study was a 12-week randomized controlled trial. Figure 1 shows the procedural flowchart of the enrollment, measurement, and data analysis performed in this study.
Figure 1.
Flowchart of the enrollment, measurement, and data analysis of the study. Abbreviations: BW, brisk walking; CP, customary pace
.
Flowchart of the enrollment, measurement, and data analysis of the study. Abbreviations: BW, brisk walking; CP, customary pace
The participants were assigned to either the group that walked 8000 steps daily, including 20 min of brisk walking at an intensity of ≥ 5 metabolic equivalents (METs) (BW group), or the group that walked 8000 steps daily at their customary pace (CP group), and were instructed to perform this daily for 3 months. The group assignment was determined using the sequential numbers assigned by the intervention instructor during recruitment, with participants randomly allocated to either the BW group or the CP group in a 1:1 ratio, alternating between the two groups. Notably, 20 min of moderate-intensity (3–5 METs) walking increases the SCFA-producing bacteria Bacteroides11; moreover, the intensity of 5 METs was set because it is the optimal intensity for young adults during moderate-intensity brisk walking. Currently, there are no established standards for the number of steps influencing the composition of intestinal bacteria. Therefore, a target of 8000 steps/day, widely recognized as beneficial for maintaining good health,12 was adopted. Participants and intervention instructors were not blinded to the assigned exercise regimen, whereas the assessors and data analysts were blinded to the group allocation.
This study spanned from the initial recruitment of participants in September 2021 to their last follow-up in June 2023. Prior to the exercise intervention, baseline measurements of body composition, physical performance, daily physical activity, and nutritional intake, and stool samples were collected. All baseline assessments were performed at least 1 week prior to the first training session. Finally, the individuals who met the selection criteria were enrolled in the BW and CP groups (n = 15), and a 12-week exercise program was initiated. Endpoints identical to those assessed at baseline were measured at least 1 week following the final session of the exercise program.
None of the participants dropped out during the intervention period and all completed the study. Participants attended all interviews with the intervention supervisor every 3 weeks during the study period.
Exercise intervention
Participants in the BW group walked 8000 steps daily, with a component of 20 minutes devoted to brisk walking at an intensity of ≥ 5 METs ( ≥ 5 METs brisk walking time), for 12 weeks. Conversely, the CP group walked 8000 steps daily at their customary pace. During the intervention period, except while sleeping and bathing, the participants wore a tri-axial accelerometer, Active style Pro HJA-750C (Omron Healthcare Co., Ltd., Kyoto, Japan), to record the number of steps, total walking time, brisk walking time at an intensity of ≥ 4 METs ( ≥ 4 METs brisk walking time), and brisk walking time at ≥ 5 METs each day. Intervention instructors reviewed the accelerometer data with the participants once every 3 weeks and provided guidance if the target numbers were not met.
Anthropometrical measurements
The participants’ body composition was assessed through bioelectrical impedance analysis using a body composition analyzer (Inbody S10; Tokyo, Japan) that estimated the percentage of fat, lean body mass, and body mass index (BMI). Quadriceps muscle strength was assessed using a manual muscle strength measuring device (#Tas F-1; Anima Corp., Tokyo, Japan), according to Hirano et al.13 Each participant performed two attempts on each leg and the maximum value of four trials was used for subsequent analyses. Cardiorespiratory fitness was assessed using the incremental shuttle walking test (ISWT) developed to assess exercise tolerance in chronic obstructive pulmonary disease, with excellent reliability and validity.14 ISWT performance is significantly correlated with peak oxygen uptake and is widely used as an index for endurance evaluation in healthy participants.15 Therefore, it was used as an index for total body endurance in this study.
Daily physical activity levels
The daily physical activity levels of participants were assessed using parameters, such as the number of steps, total waking time, ≥ 4 METs brisk walking time, and ≥ 5 METs brisk walking time. These metrics were estimated using the Active-style Pro HJA-750C (Omron Healthcare Co., Ltd. Kyoto, Japan). This device estimates METs with a tri-axial accelerometer and the time spent at a particular intensity using a validated algorithm.16,17 All participants were instructed to wear the accelerometer on their waist throughout the 1-week measurement period, except while sleeping and bathing, and to continue with daily activities as usual. The recorded data were extracted using a specialized software,16,17 and the mean daily values of all parameters recorded during the 1-week monitoring period were used for analysis.
Nutrient intake
Nutrient intake was assessed using the Food Frequency Questionnaire (FFQ) (Educational Software, Inc., Bedford, UK). The FFQ assesses the intake of key nutrients from 138 distinct food sources. This tool has been validated in various studies and has demonstrated reproducibility in measuring dietary intake.18 FFQ analysis was performed using the Kyoiku software (Tokyo, Japan). Daily energy intake and carbohydrate, protein, lipid, and dietary fiber intake were also examined.
Analysis of SCFA concentration and intestinal microbiota
The SCFA concentrations and intestinal microbiota were measured at TechnoSuruga Laboratory Co., Ltd (Shizuoka, Japan). Fecal samples were collected in a container with guanidine thiocyanate as a preservative solution (TechnoSuruga Laboratory) and were transported to the laboratory within 7 days.
The SCFA content in the feces were verified via organic acid analysis according to Takagi et al,19 with minor modifications. For determining the organic acids, 0.2 g of sample stored in Metabolokeeper® was transferred to a 2.0-mL tube with zirconia beads and suspended in Milli-Q water. The samples were heated at 85 °C for 15 minutes, vortexed at 5 m/s for 45 seconds using FastPrep-24 5G (MP Biomedicals, CA, USA), and centrifuged at 15 350 × g for 10 minutes. The supernatant was filtered. Organic acids (acetic, propionic, butyric, iso-butyric, succinic, lactic, formic, valeric, and iso-valeric acids) in the fecal samples were measured using high performance liquid chromatography (Prominence, Shimadzu, Kyoto, Japan), with a post column reaction detector (CDD-10Avp, Shimadzu), three tandemly-arranged columns (Shim-pack Fast-OA, 100 mm × 7.8 mm ID, Shimadzu), and a guard column (Shim-pack Fast-OA, 10 mm × 4.0 mm ID, Shimadzu). The system was used with a mobile phase (5 mM p-toluenesulfonic acid) and reaction solution (5 mM p-toluenesulfonic acid, 100 µM EDTA, and 20 mM Bis-Tris). The flow rate and oven temperature were 0.8 mL/min and 50°C, respectively.
The primer sequences on paired-end sequencing reads were trimmed using Cutadapt version 1.18, with default settings.20 Paired-end sequencing reads were merged using fastq-join version 1.3.1, with default settings.21 The joined amplicon sequence reads were processed using QIIME2 version 2020.63.22 Quality filtering and deletion of chimeric sequences were performed, and representative sequences were created using DADA2 (Divisive Amplicon Denoising Algorithm 2) denoise-single version 1.10.0, with default settings.23 The taxonomy of the representative sequences was assigned using the SILVA database version 13824 by training a Naive Bayes classifier.
Statistical analyses
Data are presented as means ± standard deviations. Data distribution was examined using the Shapiro–Wilk test. The baseline characteristics and daily physical activity during the intervention were compared between the groups using the unpaired t-test, except for sex, which was analyzed using the chi-square test. The effect of the exercise intervention on the clinical parameters was assessed using two-way repeated-measures analysis of variance, which was applied at multiple intervals within and between the two groups. A paired t-test was used when a significant time (intervention) effect was observed. In the event of a significant trial (group) effect, subsequent comparisons were performed using unpaired t-tests. Changes in all parameters after the intervention were assessed using an unpaired t-test. Pearson’s product-moment rank correlation coefficient test was used to examine the relationships between the parameters and changes in the relative abundance of specific types of intestinal microbiota and changes in the SCFA levels. Factors determining changes in the relative abundance of specific microbiota were identified using a stepwise regression analysis. Furthermore, post-regression analysis was performed to examine the variance inflation factor (VIF). Finally, an unpaired t-test was used to compare the changes in the relative abundance of specific types of intestinal microbiota in all participants and within the BW group with respect to the increase in daily brisk walking time. Statistical analyses were performed using the EZR statistical software (version 24.0; Saitama Medical Center, Jichi Medical University, Saitama, Japan).25 The level of significance was set at P < 0.05, and 95% confidence intervals (CIs) were calculated to estimate the strength of the association when the P values for the group comparison were significant.
Results
Clinical characteristics of the participants
Table 1 summarizes the clinical characteristics and the mean daily physical activity of both groups during the 12-week intervention period. Both groups exceeded the predetermined step count, with the BW group achieving the targeted ≥ 5 METs brisk walking time.
Table 1.
Clinical characteristics and daily physical activity levels of the participants during the intervention
|
|
Overall (n=30)
|
BW group (n=15)
|
CP group (n=15)
|
P
value
|
| Age (y) |
21.2 ± 1.0 |
21.3 ± 1.0 |
21.2 ± 1.0 |
0.855 |
| Sex, No. (%) |
|
|
|
0.027* |
| Male |
15 (50.0%) |
11 (73.3%) |
4 (26.7%) |
|
| Female |
15 (50.0%) |
4 (26.7%) |
11 (73.3%) |
|
| Height (cm) |
166.1 ± 7.2 |
169.1 ± 7.5 |
163.1 ± 5.7 |
0.021* |
| Medical history, No. (%) |
|
|
|
1.000 |
| Yes |
0 (0%) |
0 (0%) |
0 (0%) |
|
| No |
15 (0%) |
15 (0%) |
15 (0%) |
|
| Daily physical activity levels during the intervention |
|
|
|
|
| Number of steps (steps/day) |
8056 ± 1272 |
8339 ± 1409 |
7773 ± 1093 |
0.229 |
| Total walking time (min/day) |
95 ± 15 |
101 ± 11 |
89 ± 17 |
0.029* |
| Brisk walking time ( ≥ 4 METs) (min/day) |
37 ± 16 |
48 ± 13 |
26 ± 10 |
< 0.001* |
| Brisk walking time ( ≥ 5 METs) (min/day) |
15 ± 13 |
25 ± 13 |
5 ± 3 |
< 0.001* |
Abbreviations: BW, brisk walking; CP, customary pace.
Age, Hight, and Daily physical activity levels during the intervention: Values are presented as n or mean ± standard deviation (SD). *: P < 0.05 vs. BW group.
Body composition, muscle strength, physical performance, daily physical activity level, and nutrient intake following the intervention
Table 2 shows the body composition, muscle strength, physical performance, daily physical activity, and nutrient intake of both groups before and after the intervention. No significant interactions were observed for any parameters. At the baseline before the intervention, body muscle mass (P = 0.031; 95% CI, 0.34–10.86), ≥ 4 METs brisk walking time (P = 0.002, 95% CI 8.05–29.02), and ≥ 5 METs brisk walking time (P = 0.002; 95% CI, 4.29–19.16) in the BW group were significantly higher than those in the CP group. After the intervention, shuttle distance (P = 0.030; 95% CI, 6.26–232.41), ≥ 4 METs brisk walking time (P = 0.019; 95% CI, 3.73–30.27), and ≥ 5 METs brisk walking time (p = 0.005; 95% CI, 5.80–29.27), were significantly higher in the BW group than those in the CP group. The degree of change from the intervention did not differ between the two groups for any of the other parameters. In both groups, the intervention significantly increased the ≥ 4 METs brisk walking time (BW group: P = 0.026; 95% CI, -25.94–0.29; CP group: p = 0.002; 95% CI, -22.83–6.46); moreover, in the CP group, post-intervention results showed a significant increase in shuttle distance (P = 0.004; 95% CI, -93.37–23.96) and ≥ 5 METs brisk walking time (P = 0.045; 95% CI, -7.17–0.69). No changes were observed in the other variables, including total energy and nutrient intakes, due to the intervention in either group. Protein content was higher in the BW group than that in the CP group before and after the intervention, but the change due to the intervention did not differ between the two groups (BW, -1.08 ± 2.78; CT, -0.13 ± 3.60; p = 0.424; 95% CI, -3.36–1.45).
Table 2.
Changes in the parameters following the intervention
|
|
BW group (n=15)
|
CP group (n=15)
|
|
Baseline
|
Post
|
Baseline
|
Post
|
| Weight (kg) |
64.7 ± 9.4 |
65.2 ± 10.1 |
61.4 ± 13.1 |
61.4 ± 12.6 |
| BMI (kg/m2) |
22.6 ± 2.4 |
22.7 ± 2.5 |
23.0 ± 3.9 |
23.0 ± 3.9 |
| Body fat (%) |
21.7 ± 6.7 |
22.9 ± 8.1 |
26.5 ± 8.2 |
26.3 ± 8.2 |
| Total muscle mass (kg) |
47.7 ± 7.1 |
47.2 ± 8.0 |
42.1 ± 6.9** |
41.5 ± 7.4 |
| Quad. muscle strength (kg) |
56.2 ± 18.5 |
57.4 ± 19.0 |
45.6 ± 11.9 |
46.2 ± 11.1 |
| ISWT (m) |
690.7 ± 179.8 |
740.0 ± 160.5 |
562.0 ± 135.7 |
620.7 ± 141.2*,** |
| Daily physical activity |
|
|
|
|
| Number of steps (steps/day) |
7490 ± 1295 |
8480 ± 2258 |
7177 ± 2461 |
8208 ± 1325 |
| Total walking time (min/day) |
94 ± 17 |
103 ± 25 |
90 ± 32 |
93 ± 18 |
| Brisk walking time ( ≥ 4 METs), (min/day) |
36 ± 16 |
49 ± 21* |
17 ± 12** |
32 ± 14*,** |
| Brisk walking time ( ≥ 5 METs), (min/day) |
15 ± 13 |
24 ± 21 |
3 ± 4** |
7 ± 6** |
| Nutrient intake |
|
|
|
|
| Total energy (kcal/day) |
2031 ± 170 |
1995 ± 205 |
1891 ± 153 |
1898 ± 179 |
| Carbohydrates (g/day) |
270.9 ± 46.1 |
263.4 ± 50.0 |
247.3 ± 19.2 |
252.8 ± 27.3 |
| Protein (g/day) |
81.7 ± 5.2 |
80.6 ± 6.9 |
75.0 ± 7.4** |
74.9 ± 7.6** |
| Lipid (g/day) |
61.6 ± 2.3 |
60.6 ± 3.2 |
59.3 ± 4.2 |
58.9 ± 4.0 |
| Fiber (g/day) |
16.8 ± 1.4 |
16.3 ± 1.4 |
16.8 ± 0.8 |
16.4 ± 0.7 |
Abbreviations: BW, brisk walking; CP, customary pace; BMI, body mass index; Quad. muscle strength, Quadriceps muscle strength;
ISWT, incremental shuttle walking test.
Values are presented as n or means ± SDs. *P < 0.05 within the group. **P < 0.05 between the groups.
Fecal short-chain fatty acid concentration
The fecal concentrations of acetic, propionic, butyric, isobutyric, succinic, isovaleric, valeric, lactic, and formic acid were measured. The detection rates of these SCFAs in the participants at the baseline were 100.0%, 96.7%, 96.7%, 56.7%, 73.3%, 0%, 0%, 46.7%, and 63.3%, respectively. After the intervention, the detection rates were 100.0%, 100.0%, 100.0%, 43.3%, 73.3%, 20.2%, 3.3%, 46.7%, and 56.7%, respectively. Table 3 presents the results of the study. No interactions were observed for any SCFA. After the intervention, acetic acid concentration increased in both groups, with a significant increase observed in the BW group (P = 0.030; 95% CI, -1.34–0.08). The concentrations of other SCFAs did not change before or after exercise in either group. The degree of change due to the intervention did not differ between the two groups for all SCFAs.
Table 3.
Fecal short-chain fatty acids/organic acid concentrations
|
|
BW group (n=15)
|
CP group (n=15)
|
P
value
|
|
Baseline
|
Post
|
Baseline
|
Post
|
Main effect of intervention
|
Main effect of group
|
Intervention×group interaction
|
| Organic acids |
|
|
|
|
|
|
|
| Acetic acid |
1.979 ± 0.699 |
2.688 ± 1.264* |
2.012 ± 0.962 |
2.839 ± 1.440 |
0.004 |
0.783 |
0.814 |
| Propionic acid |
1.033 ± 0.367 |
1.164 ± 0.630 |
0.868 ± 0.510 |
1.165 ± 0.528 |
0.115 |
0.550 |
0.535 |
| Butyric acid |
0.906 ± 0.460 |
1.121 ± 0.801 |
0.825 ± 0.469 |
1.002 ± 0.599 |
0.109 |
0.591 |
0.871 |
| Iso-butyric acid |
0.106 ± 0.116 |
0.078 ± 0.118 |
0.099 ± 0.104 |
0.122 ± 0.141 |
0.922 |
0.607 |
0.357 |
| Succinic acid |
0.193 ± 0.475 |
0.381 ± 1.020 |
0.419 ± 0.666 |
0.319 ± 0.827 |
0.803 |
0.751 |
0.420 |
| Iso-valeric acid |
0.162 ± 0.198 |
0.160 ± 0.219 |
0.147 ± 0.140 |
0.207 ± 0.187 |
0.435 |
0.790 |
0.411 |
| n-valeric acid |
0.119 ± 0.224 |
0.139 ± 0.209 |
0.131 ± 0.178 |
0.130 ± 0.143 |
0.684 |
0.983 |
0.671 |
| Lactic acid |
0.000 ± 0.000 |
0.064 ± 0.149 |
0.000 ± 0.000 |
0.018 ± 0.054 |
0.055 |
0.267 |
0.267 |
| Formic acid |
0.000 ± 0.000 |
0.011 ± 0.043 |
0.000 ± 0.000 |
0.000 ± 0.000 |
0.326 |
0.326 |
0.326 |
Abbreviations: BW, brisk walking; CP, customary pace.
Values are presented as n or mean ± SD. *: P < 0.05 within the group. **: P < 0.05 between the groups.
Composition of intestinal microbiota
Figure 2 shows the abundance of the major bacteria detected from the phylum to the species level in the composition of the intestinal microbiota in both groups. After the intervention, the relative abundance of Negativicutes at the class level (P = 0.015; 95% CI, -2.44–0.31), Veillonellales at the order level (P = 0.011; 95% CI, -1.51–0.23), Anaerostipes at the genus level (P = 0.008, 95% CI, -1.72–0.31), and Anaerostipes hadrus at the species level (P = 0.009; 95% CI, -1.71–0.29) were significantly higher in the BW group than that in the CP group. The acetate-producing bacteria, Bifidobacterium at the genus level, increased only in the BW group after the intervention; however, the difference was not statistically significant (P = 0.114; 95% CI, -9.12–1.10).
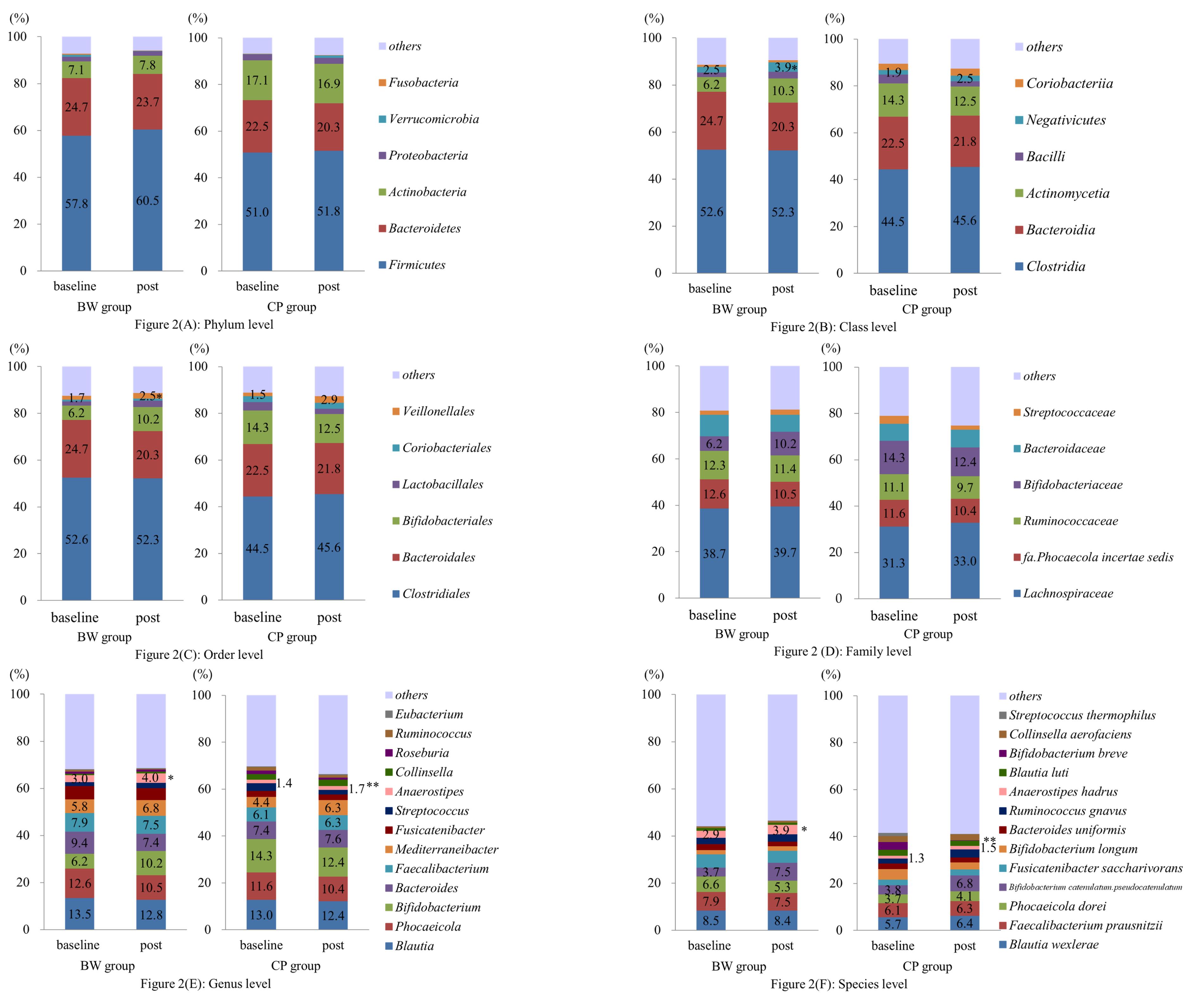
Figure 2.
Changes in the composition of the intestinal microbiota following the intervention from the phylum level to the species level (A–F). The relative abundance of Intestinal Negativicutes at class level (B), Veillonellales at order level (C), Anaerostipes at genus level (E), and Anaerostipes hadrus at species level (F) were significantly higher in the BW group than that in the CP group. * P < 0.05, compared to baseline. Abbreviations: BW, brisk walking; CP, customary pace
.
Changes in the composition of the intestinal microbiota following the intervention from the phylum level to the species level (A–F). The relative abundance of Intestinal Negativicutes at class level (B), Veillonellales at order level (C), Anaerostipes at genus level (E), and Anaerostipes hadrus at species level (F) were significantly higher in the BW group than that in the CP group. * P < 0.05, compared to baseline. Abbreviations: BW, brisk walking; CP, customary pace
Relationship between changes in the parameters and change in acetic acid levels and relative abundance of intestinal Bifidobacterium after intervention
We examined the relationships between the change in the ≥ 4 METs brisk walking time (Δ ≥ 4 METs brisk walking time), total walking time, ≥ 4 METs brisk walking time, and ≥ 5 METs brisk walking time during intervention, and the change in acetic acid (Δacetic acid) and the relative intestinal abundance of Bifidobacterium (Δ%Bifidobacterium). No significant correlations were observed between these parameters and acetic acid levels in the BW group (Table 3). A significant positive correlation was found between ≥ 5 METs brisk walking time during the intervention and Δ%Bifidobacterium when analyzing all the participants (r = 0.443, P =0.014) (Table 4). To identify the factors that contribute to Δ%Bifidobacterium, we performed a stepwise multiple regression analysis with Δ%Bifidobacterium as the dependent variable. Independent variables included brisk walking time ≥ 5 METs during the intervention, as well as changes in ISWT distance and dietary fiber, which reportedly affects SCFA-producing bacteria.7,11 In the present study, ≥ 5 METs brisk walking time during the intervention was an independent contributor (B = 0.327, P = 0.014). The adjusted coefficient of determination for degrees of freedom was 0.12. The VIF was < 2 for all variables, indicating no multicollinearity issues.
Table 4.
Correlation coefficients in simple regression analysis between Δ%Bifidobacterium and physical activity levels in all participants and the BW group
|
Related physical activity level
|
Overall
|
BW group
|
|
Correlation coefficient
|
P
value
|
95% CI
|
Correlation coefficient
|
P
value
|
95% CI
|
| Δ ≥ 4 METs brisk walking time |
-0.299 |
0.109 |
-0.59–0.07 |
-0.256 |
0.356 |
-0.68–0.29 |
| Total walking time during the intervention |
0.130 |
0.495 |
-0.24–0.47 |
-0.290 |
0.294 |
-0.70–0.65 |
| ≥ 4 METs brisk walking time during the intervention |
0.307 |
0.099 |
-0.06–0.60 |
0.330 |
0.230 |
-0.22–0.26 |
| ≥ 5 METs brisk walking time during the intervention |
0.443 |
0.014* |
0.10–0.69 |
0.413 |
0.126 |
-0.12–0.76 |
Abbreviations: BW, brisk walking; CI, confidence interval.
*P < 0.05.
Impact of brisk walking at an intensity of ≥ 5 METs on changes in the relative abundance of intestinal Bifidobacterium following intervention
We examined the effect of ≥ 5 METs brisk walking on Δ%Bifidobacterium. All participants and participants in the BW group were allocated into two groups based on whether they performed ≥ 5 METs brisk walking for > 20 minutes or < 20 minutes during the intervention. The Δ%Bifidobacterium was greater in participants who engaged in brisk walking for ≥ 20 minutes daily than that in those who walked for < 20 minutes daily (Figure 3).
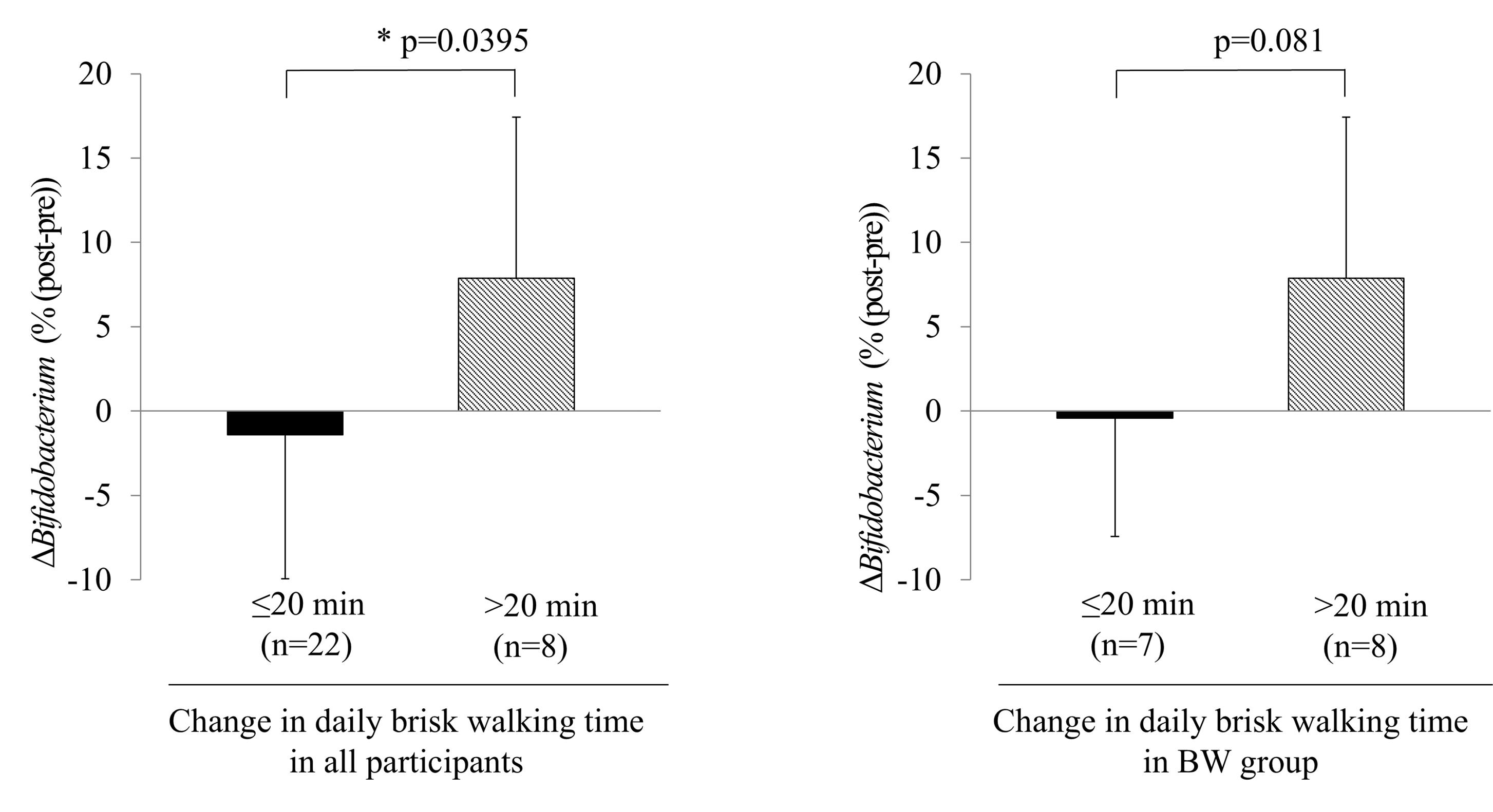
Figure 3.
Analyzing the participants revealed that the Δ%Bifidobacterium in those who engaged in brisk walking for ≥ 20 minutes daily was greater than that in those who walked for < 20 min. * P < 0.05, compared to baseline. Abbreviations: BW, brisk walking
.
Analyzing the participants revealed that the Δ%Bifidobacterium in those who engaged in brisk walking for ≥ 20 minutes daily was greater than that in those who walked for < 20 min. * P < 0.05, compared to baseline. Abbreviations: BW, brisk walking
Discussion
In this study, we aimed to examine whether increasing daily physical activity increases the SCFA levels in the intestines of healthy young adults. The results showed that 20 minutes of ≥ 5 METs brisk walking per day may increase the amount of acetic acid, a type of SCFA, via increasing the relative abundance in Bifidobacterium, an acetic acid-producing bacterium.
Physical exercise alters the composition of the intestinal bacteria,14 through SCFA-producing bacteria26; however, no consensus exists on the relevance of this relationship. Young adults with high cardiorespiratory fitness have a higher amount and number of butyrate-producing bacteria, such as Clostridiales spp. and Roseburia spp, in the feces.26 Notably, 6 weeks of moderate aerobic exercise training in young women with BMI < 25 kg/m2 increased SCFA concentration, with the contribution of the SCFA-producing bacteria,Faecalibacterium spp. and Lachnospira spp27; moreover, in active older individuals with insomnia, the concentration of SCFA in feces was found to be low, with a negative relationship between the number of steps and the SCFA propionic acid.28 A study on rodents reported that 6 days of rotational running exercise increased the Bifidobacterium levels.29 Women with active lifestyles had higher levels of Bifidobacterium,30 and in middle-aged men, 24 weeks of exercise increased the Bifidobacterium and fecal butyrate levels.31 Furthermore, navy trainees who underwent 8 weeks of physical exercise, including military training and cardio- and weight-training, showed increased Bifidobacterium levels.32 In contrast, a study comparing bodybuilders, elite distance runners, and healthy men reported that Bifidobacterium abundance was the lowest in bodybuilders with exercise habits.33 These results are consistent with those of previous studies on aerobic exercise.
Currently, the mechanisms by which aerobic exercise increases the number of SCFA-producing bacteria are not clearly understood. The positive effects of endurance exercise on the gut include increased mitochondrial resynthesis and reactive oxygen species, reduced gut inflammation due to decreased intestinal permeability, suppression of oxidative stress, and increased intestinal blood flow, which improve the gut microbiota.34,35 Scheiman et al also noted an increase in Veillonella in runners after a marathon and reported that exercise may increase Veillonella, an exercise-induced, lactic acid-fed, SCFA-producing bacterium, which, in turn, increases propionate, an SCFA.36 This finding suggests that exercise-induced substances may affect SCFA-producing bacteria. Notably, a study that confirmed the movement of intestinal microbiota and glucose in patients with diabetes receiving metformin, revealed increased Bifidobacterium compared to non-users, which was attributed to a higher accumulation of Bifidobacterium’s energy source, glucose, in the ileum and colon.37 Therefore, aerobic exercise may affect Bifidobacterium,38 which feeds on sugar produced during metabolism. However, no studies have shown a relationship between sugar metabolism and intestinal bacteria during exercise; therefore, further verification is needed.
Bifidobacterium, the focus of this study, plays an important role in human health by producing SCFAs, which can improve hypercholesterolemia39 and diabetes,40 prevent infectious diseases, improve immune response,41 and suppress the onset of depression.42 Furthermore, it is involved in sarcopenia, causing muscle loss.43 As daily exercise helps maintain health by improving the intestinal environment, we suggest that it is important to develop an exercise habit at a young age.
This study had some limitations. First, this study included both sexes owing to the small sample size and the fact that it was a randomized trial. Although the present study design was adopted because it has been reported that the composition of the gut microbiota does not differ significantly between men and women, exercise functions of men and women differ, which affects the clinical outcomes of body composition and cardiopulmonary exercise function. Therefore, caution is needed in interpreting the results comparing these factors in the two groups. Second, the ISWT, used to assess cardiorespiratory function, may underestimate exercise tolerance if participants fail to reach maximum walking speed. Although objective assessments, such as expiratory gas analysis, were impossible due to COVID-19 infection issues, future studies should accurately assess cardiopulmonary function.
Conclusion
Daily 20-minute sessions of brisk walking at ≥ 5 METs may increase the SCFA levels, particularly acetic acid, by promoting an increase in the intestinal Bifidobacterium levels. Although it is important to increase daily physical activity from a young age to improve the intestinal environment, further research is needed to clarify the mechanism by which exercise increases Bifidobacterium abundance and SCFA production.
Acknowledgments
We are grateful to the TechnoSuruga Laboratory for data analysis. We would also like to thank Editage (https://www.editage.jp/) for the English language editing.
Competing Interests
The authors declare that they have no competing interests.
Ethical Approval
This study was approved by the Research Ethics Committee of Aino University, Japan (approval no. 2021-005).
References
- Chen Y, Zhou J, Wang L. Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol 2021; 11:625913. doi: 10.3389/fcimb.2021.625913 [Crossref] [ Google Scholar]
- Bouter KE, van Raalte DH, Groen AK, Nieuwdorp M. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology 2017; 152(7):1671-8. doi: 10.1053/j.gastro.2016.12.048 [Crossref] [ Google Scholar]
- Wu W, Sun M, Chen F, Cao AT, Liu H, Zhao Y. Microbiota metabolite short-chain fatty acid acetate promotes intestinal IgA response to microbiota which is mediated by GPR43. Mucosal Immunol 2017; 10(4):946-56. doi: 10.1038/mi.2016.114 [Crossref] [ Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun 2013; 4:1829. doi: 10.1038/ncomms2852 [Crossref] [ Google Scholar]
- Frost G, Sleeth ML, Sahuri-Arisoylu M, Lizarbe B, Cerdan S, Brody L. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun 2014; 5:3611. doi: 10.1038/ncomms4611 [Crossref] [ Google Scholar]
- Shimizu H, Ohue-Kitano R, Kimura I. Regulation of host energy metabolism by gut microbiota-derived short-chain fatty acids. Glycative Stress Research 2019; 6(3):181-91. doi: 10.24659/gsr.6.3_181 [Crossref] [ Google Scholar]
- Dziewiecka H, Buttar HS, Kasperska A, Ostapiuk-Karolczuk J, Domagalska M, Cichoń J. Physical activity induced alterations of gut microbiota in humans: a systematic review. BMC Sports Sci Med Rehabil 2022; 14(1):122. doi: 10.1186/s13102-022-00513-2 [Crossref] [ Google Scholar]
- Motiani KK, Collado MC, Eskelinen JJ, Virtanen KA, Löyttyniemi E, Salminen S. Exercise training modulates gut microbiota profile and improves endotoxemia. Med Sci Sports Exerc 2020; 52(1):94-104. doi: 10.1249/mss.0000000000002112 [Crossref] [ Google Scholar]
- Hampton-Marcell JT, Eshoo TW, Cook MD, Gilbert JA, Horswill CA, Poretsky R. Comparative analysis of gut microbiota following changes in training volume among swimmers. Int J Sports Med 2020; 41(5):292-9. doi: 10.1055/a-1079-5450 [Crossref] [ Google Scholar]
- Karl JP, Margolis LM, Madslien EH, Murphy NE, Castellani JW, Gundersen Y. Changes in intestinal microbiota composition and metabolism coincide with increased intestinal permeability in young adults under prolonged physiological stress. Am J Physiol Gastrointest Liver Physiol 2017; 312(6):G559-71. doi: 10.1152/ajpgi.00066.2017 [Crossref] [ Google Scholar]
- Morita E, Yokoyama H, Imai D, Takeda R, Ota A, Kawai E. Aerobic exercise training with brisk walking increases intestinal bacteroides in healthy elderly women. Nutrients 2019; 11(4):868. doi: 10.3390/nu11040868 [Crossref] [ Google Scholar]
- Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health 2022; 7(3):e219-28. doi: 10.1016/s2468-2667(21)00302-9 [Crossref] [ Google Scholar]
- Hirano M, Katoh M, Gomi M, Arai S. Validity and reliability of isometric knee extension muscle strength measurements using a belt-stabilized hand-held dynamometer: a comparison with the measurement using an isokinetic dynamometer in a sitting posture. J Phys Ther Sci 2020; 32(2):120-4. doi: 10.1589/jpts.32.120 [Crossref] [ Google Scholar]
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992; 47(12):1019-24. doi: 10.1136/thx.47.12.1019 [Crossref] [ Google Scholar]
- Agarwal B, Shah M, Andhare N, Mullerpatan R. Incremental shuttle walk test: reference values and predictive equation for healthy Indian adults. Lung India 2016; 33(1):36-41. doi: 10.4103/0970-2113.173056 [Crossref] [ Google Scholar]
- Oshima Y, Kawaguchi K, Tanaka S, Ohkawara K, Hikihara Y, Ishikawa-Takata K. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture 2010; 31(3):370-4. doi: 10.1016/j.gaitpost.2010.01.005 [Crossref] [ Google Scholar]
- Ohkawara K, Oshima Y, Hikihara Y, Ishikawa-Takata K, Tabata I, Tanaka S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr 2011; 105(11):1681-91. doi: 10.1017/s0007114510005441 [Crossref] [ Google Scholar]
- Tsubono Y, Takamori S, Kobayashi M, Takahashi T, Iwase Y, Iitoi Y. A data-based approach for designing a semiquantitative food frequency questionnaire for a population-based prospective study in Japan. J Epidemiol 1996; 6(1):45-53. doi: 10.2188/jea.6.45 [Crossref] [ Google Scholar]
- Takagi T, Kunihiro T, Takahashi S, Hisada T, Nagashima K, Mochizuki J. A newly developed solution for the preservation of short-chain fatty acids, bile acids, and microbiota in fecal specimens. J Clin Biochem Nutr 2023; 72(3):263-9. doi: 10.3164/jcbn.22-107 [Crossref] [ Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 2011; 17(1):10-2. doi: 10.14806/ej.17.1.200 [Crossref] [ Google Scholar]
- Aronesty E. Comparison of sequencing utility programs. The Open Bioinformatics Journal 2013; 7(1):1-8. doi: 10.2174/1875036201307010001 [Crossref] [ Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 2019; 37(8):852-7. doi: 10.1038/s41587-019-0209-9 [Crossref] [ Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 2016; 13(7):581-3. doi: 10.1038/nmeth.3869 [Crossref] [ Google Scholar]
- Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41(Database issue):D590-6. doi: 10.1093/nar/gks1219 [Crossref] [ Google Scholar]
- Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48(3):452-8. doi: 10.1038/bmt.2012.244 [Crossref] [ Google Scholar]
- Estaki M, Pither J, Baumeister P, Little JP, Gill SK, Ghosh S. Cardiorespiratory fitness as a predictor of intestinal microbial diversity and distinct metagenomic functions. Microbiome 2016; 4(1):42. doi: 10.1186/s40168-016-0189-7 [Crossref] [ Google Scholar]
- Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA. Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 2018; 50(4):747-57. doi: 10.1249/mss.0000000000001495 [Crossref] [ Google Scholar]
- Magzal F, Shochat T, Haimov I, Tamir S, Asraf K, Tuchner-Arieli M. Increased physical activity improves gut microbiota composition and reduces short-chain fatty acid concentrations in older adults with insomnia. Sci Rep 2022; 12(1):2265. doi: 10.1038/s41598-022-05099-w [Crossref] [ Google Scholar]
- Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One 2013; 8(5):e65465. doi: 10.1371/journal.pone.0065465 [Crossref] [ Google Scholar]
- Bressa C, Bailén-Andrino M, Pérez-Santiago J, González-Soltero R, Pérez M, Montalvo-Lominchar MG. Differences in gut microbiota profile between women with active lifestyle and sedentary women. PLoS One 2017; 12(2):e0171352. doi: 10.1371/journal.pone.0171352 [Crossref] [ Google Scholar]
- Erlandson KM, Liu J, Johnson R, Dillon S, Jankowski CM, Kroehl M. An exercise intervention alters stool microbiota and metabolites among older, sedentary adults. Ther Adv Infect Dis 2021; 8:20499361211027067. doi: 10.1177/20499361211027067 [Crossref] [ Google Scholar]
- Jung Y, Tagele SB, Son H, Ibal JC, Kerfahi D, Yun H. Modulation of gut microbiota in Korean navy trainees following a healthy lifestyle change. Microorganisms 2020; 8(9):1265. doi: 10.3390/microorganisms8091265 [Crossref] [ Google Scholar]
- Jang LG, Choi G, Kim SW, Kim BY, Lee S, Park H. The combination of sport and sport-specific diet is associated with characteristics of gut microbiota: an observational study. J Int Soc Sports Nutr 2019; 16(1):21. doi: 10.1186/s12970-019-0290-y [Crossref] [ Google Scholar]
- Clark A, Mach N. The crosstalk between the gut microbiota and mitochondria during exercise. Front Physiol 2017; 8:319. doi: 10.3389/fphys.2017.00319 [Crossref] [ Google Scholar]
- Keirns BH, Koemel NA, Sciarrillo CM, Anderson KL, Emerson SR. Exercise and intestinal permeability: another form of exercise-induced hormesis?. Am J Physiol Gastrointest Liver Physiol 2020; 319(4):G512-8. doi: 10.1152/ajpgi.00232.2020 [Crossref] [ Google Scholar]
- Scheiman J, Luber JM, Chavkin TA, MacDonald T, Tung A, Pham LD. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019; 25(7):1104-9. doi: 10.1038/s41591-019-0485-4 [Crossref] [ Google Scholar]
- Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Mannerås-Holm L. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med 2017; 23(7):850-8. doi: 10.1038/nm.4345 [Crossref] [ Google Scholar]
- Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 2001; 81(3):1031-64. doi: 10.1152/physrev.2001.81.3.1031 [Crossref] [ Google Scholar]
- Rerksuppaphol S, Rerksuppaphol L. A randomized double-blind controlled trial of Lactobacillus acidophilus plus bifidobacterium bifidum versus placebo in patients with hypercholesterolemia. J Clin Diagn Res 2015; 9(3):KC01-4. doi: 10.7860/jcdr/2015/11867.5728 [Crossref] [ Google Scholar]
- Sharma P, Bhardwaj P, Singh R. Administration of Lactobacillus casei and bifidobacterium bifidum ameliorated hyperglycemia, dyslipidemia, and oxidative stress in diabetic rats. Int J Prev Med 2016; 7:102. doi: 10.4103/2008-7802.188870 [Crossref] [ Google Scholar]
- Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des 2009; 15(13):1428-518. doi: 10.2174/138161209788168155 [Crossref] [ Google Scholar]
- Aizawa E, Tsuji H, Asahara T, Takahashi T, Teraishi T, Yoshida S. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord 2016; 202:254-7. doi: 10.1016/j.jad.2016.05.038 [Crossref] [ Google Scholar]
- Liu C, Cheung WH, Li J, Chow SK, Yu J, Wong SH. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle 2021; 12(6):1393-407. doi: 10.1002/jcsm.12784 [Crossref] [ Google Scholar]